Before growing, forming beds and planning future plantings, it is good to know the acidity level of your land. This will help to correctly distribute forces and funds for its development. The acidity of the soil, along with its composition and the level of groundwater, is an important indicator that tells us whether it is worth growing a particular crop on the site. Just as different people have their own preferences regarding food, plants have their own preferences regarding soil acidity.
Soil deoxidation
The composition of the soil is different everywhere, and the acidity level also varies, which has a direct impact on the survival rate of plants. Acidic soil is characterized by the transition of microelements into various states, due to which many of them become difficult to access for plants, while others acquire a toxic form. So, if you plant an unadapted plant in acidic soil, you can observe signs of chlorosis and a lack of growth and flowering. Such soils are heavier, have poorer air permeability, and often become waterlogged.
Important! How to measure soil acidity using instruments and available tools can be read in this material.
Weeds that grow most actively can tell you how acidic the soil is. These are the so-called acidophiles - plants of acidic soils. If you diligently control sedge in your area, mosses thrive in the shade, and plantains, horsetails and horse sorrel multiply in the sun, probably a pH indicator. in the area is reduced.
If the ph level. below 5, then such soil requires deoxidation. You can adjust the indicators using fertilizers and organic matter. Read more…
- It is advisable to choose calcium and phosphorus mixtures as fertilizers, since it is these microelements that plants in an acidic environment are sorely lacking. Good results are achieved by adding calcium nitrate, or superphosphate. Other deficient microelements include manganese, molybdenum, magnesium, and copper. But keep in mind that a visible effect can only be achieved over time if you regularly replenish the mineral reserves of the soil. How and when to fertilize? The article garden fertilizers will answer these questions.
- If you are just planning to create a flowerbed or bed on your site, you can prepare the soil by planting green manure - crops that improve the structure of the soil and enrich it with nitrogen. For acidic soils these are: legumes, rye, oats, vetch, phacelia. Once they have served their purpose, they can be used as mulch.
- Soil deoxidation by isolating - adding ash is considered one of the safest and most useful methods. In addition to alkalization, ash itself is a source of valuable elements. Application rate – 0.2 kg. 1 sq.m.
- To improve acidic soil, liming is used - adding slaked fluff lime to the surface layer of soil. This method is suitable for heavy clay soils, and even then only for digging. The approximate application rate is 0.2-0.5 kg. 1 sq.m. depending on the acidity level.
- If deoxidation is carried out on light sandy soil, you can use dolomite flour and chalk, which, in addition to alkalization, enrich the soil with calcium and magnesium. Application rate – 0.2-0.3 kg. 1 sq.m.
✿
How to deal with alkalization
You cannot get a high yield on alkaline soil. This soil is suitable for few crops, so you should try to bring it closer to neutral. This is done by applying organic and mineral fertilizers, but organics should come first.
To increase the acidity level by 1 pH unit for each square meter of area, you need to add 3 kg of manure or 9 kg of compost. You can also add sphagnum peat.
You should be careful when applying mineral fertilizers, because they are highly concentrated and their correct dose is difficult to calculate. If an error occurs, it is very difficult to correct and may take years.
A good effect is obtained by watering the soil with oxalic or citric acid, adding 2 to 5 grams of the substance to a 10-liter bucket. You can also use regular or 9% apple cider vinegar for this, diluting 100 grams of it per 10 liters. All work is carried out in the fall.
If you correctly apply knowledge about soil acidity, you can get a rich harvest of garden crops every year without much effort.
The effect of acidity on yields
Acidity affects the yield of vegetable crops in different ways. Most of them prefer a slightly acidic or neutral soil reaction. Many wild berries, such as blueberries, are the biggest lovers of acid - they will not grow in slightly acidic, much less neutral, soil.
Tomatoes, pumpkins, sorrel and parsley need slightly less acidic soil. For example, potatoes respond well to slightly acidic soil, and melons such as zucchini, cucumbers, legumes, as well as radishes and radishes. A neutral environment is necessary for beets, garlic, onions and cabbage.
Beautiful ornamental plants - rhododendrons - are also record holders for acidity. For them, it is necessary to specially prepare the soil in the place where you plan to grow them and additionally acidify the bed every year.
Why is acidity so dangerous? The fact is that the main nutrients - the NPK complex - are not available to the root system, which is why dwarfism appears in the aerial parts and fruits. At the same time, high concentrations of aluminum and manganese further reduce the ability of crops to absorb nutrients.
The supply of nitrogen, potassium and phosphorus decreases, and with it the immunity of plants. In acidic soil, they get sick more often and are susceptible to epidemics of fungal infections.
Plants soil pH indicators
There is no need to go anywhere if you know the indicator plants already present in the beds; as a rule, they are weeds, and you have to constantly fight with them.
- So if you notice a predominance in the beds: horsetails, sorrel, mosses, buttercups, oxalis - the environment is acidic; heathers, cornflowers, forest ferns – slightly acidic;
- cornflowers, common chicory, types of thistles, wheatgrass, shepherd's bag, feather grass, cloves, bluebells, etc. - grow on neutral soils;
— species of alfalfa, thyme, bindweed, poppy, sedge, etc. – alkaline.
Cultivated plants basically love neutral soils.
— Now it’s worth breaking down the cultivated plants according to preferences: acidic soils - blueberries;
— slightly acidic ones are preferred by raspberries, strawberries, wild strawberries, honeysuckle, serviceberry, apple trees (tolerant), cranberries, tomatoes, potatoes, roses, hydrangeas, heather, etc.;
- neutral - irreplaceable for cabbage, beets, garlic, onions, cucumbers, bell peppers, currants, cherries, plums, apple trees.
Soil acidity level
The acidity of the soil is affected by the amount and composition of chemical elements. The level of acidity is indicated by the pH symbol. The pH value depends on the amount and composition of chemical elements in the soil. Based on the results of chemical experiments, it was established that nutrients are optimally available to vegetable and horticultural crops at pH = 6.0...7.0. A soil pH of 7.0 is considered neutral.
All values below 7.0 are considered acidic and the lower the number, the higher the acidity. Like acidity, biological processes in plants are also affected by alkalinity, which is caused by alkaline elements contained in the soil. Alkalinity is reflected in pH values above 7.0 units (Table 1).
These and other deviations from the neutral indicator indicate the degree of availability of certain elements to plants, which can decrease or, conversely, increase so much that the nutrients become toxic and the plant dies.
Table 1. Types of soils by degree of acidity
| Soil acidity level | pH, units | Soil types |
| strongly acidic | 3,5 – 4,5 | bog soils, lowland peat |
| sour | 4,6 – 5,3 | peaty, coniferous, clay-turf |
| slightly acidic | 5,4 – 6,3 | heather, turf |
| neutral | 6,4 – 7,3 | turf, humus, deciduous |
| weakly alkaline | 7,4 – 8,0 | carbonate |
| alkaline | 8,1 – 8,5 | carbonate |
| highly alkaline | 8,5 – 9,0 | carbonate |
Determination of soil acidity and its deoxidation
Types of soils and their acidity
The level of soil acidity directly depends on the presence of mineral and organic acids, as well as colloids with acidic characteristics.
Without bothering your head with abstruse terms, it is necessary to explain in your fingers what types of soils you have to work with and how they affect green organisms.
The relationship of hydrogen ions, the so-called H-ions, located in soil moisture (in the liquid phase), with aluminum and exchangeable hydrogen ions in the soil absorption complex (SAC) characterizes the acidity of the soil material.
An ignorant gardener should explain the information about the absorption complex.
PPC is a set of highly dispersed organic, mineral, organomineral formations insoluble in an aqueous environment, i.e. the physical size of suspended particles, which, in turn, are capable of exchanging and absorbing free ions.
The following types of soil acidity have been defined in the scientific community:
- current.
- potential.
- exchange.
In addition, there is also the concept of hydrolytic acidity - this is a total component that takes into account exchangeable and actual. Its final value is used to determine the amount of lime during liming.
Take my word for it that the key phrase in the previous paragraph is “academic environment.” For in applied gardening these names are not used at all. And summer residents are least interested in deciphering these terms.
They are interested in the hydrogen index or acidity index, which is usually denoted pH.
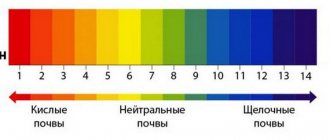
The digital values of this indicator are displayed on a scale that is graduated from 1 to 14 units. A higher pH value indicates a lower content of H-ions and vice versa.
There are natural indicator plants that can be used to determine the amount of acids in the soil. But few people know that plant crops, by their presence, already indicate the condition of the soil.
| Natural environment | Soil characteristics | Acidity level (pH) |
| Wetland and dry wetland | Strongly acidic | 3–4,5 |
| Mostly coniferous forests | Sour | 4–4,5 |
| Mixed forests | Medium acid | 4,6–6 |
| Deciduous forests | Weak acid | 5 –6,4 |
| water meadows | Neutral | 6–7 |
| Steppe zone | Neutral to alkaline | 7–8 |
What weeds grow in acidic soils + photo
Experts have long noticed that different weeds grow at different pH levels, but there are those that can adapt to any.
There are several types of weeds that adhere to different levels of soil acidity:
- extreme acidophiles that grow in highly acidic soil (pH 3 – 4);
- medium acidophiles, grow in slightly acidic soil;
- weak acidophiles take root in areas with neutral and slightly acidic pH;
- neutrophils, soil with a neutral pH is acceptable for growth and reproduction.
List and photos of weeds that are an indicator of acidic, slightly acidic soil.
- Sour sorrel (common or horse sorrel) is a perennial plant distributed throughout Russia. It has long-petioled basal leaves, entire-edged, elongated-oval.
- Horsetail is a perennial plant with a leafless stem and leaf teeth collected in whorls with oval-shaped spikelets. Grows up to 50 cm.
- Whitebeard protruding, it includes a number of plants belonging to the Poa and Poaceae families. The leaves are bristly, up to 15 cm long, and the inflorescence is a spikelet.
- Wild mint (field mint, meadow mint) is a member of the Lamiaceae family, the leaves are elongated-oval, jagged along the edges, aromatic, located on short petioles of green stems growing up to 40 cm.
- Tricolor violet (Pansy, Ivan da Marya) - narrow lance-shaped leaves, wide oval lower ones, five-petalled blue-violet flowers with a yellow center.
- Field navel is a weed of the Asteraceae family, flowers similar to medicinal chamomile, leaves openwork, pinnately dissected, short, oblong.
- Crowberry - Heather family, other names: Shiksha, Ernik, Voronika, Bagnovka. A perennial creeping plant up to 1 m in length. The leaves are watery, alternate, narrowly elliptical in shape, similar to a Christmas tree. The fruit is a black berry, spherical, flattened in shape.
- Meadow grass is a family of Broomrapaceae, an annual plant. The leaves are lanceolate, pointed, entire, up to 9 cm in length. On the stem are single or collected in inflorescences yellow flowers, similar to “dogs, helmets”.
- Marsh wild rosemary - family Ericaceae, leaves on short petioles, elongated-elliptical, alternate. The flowers are collected in inflorescences shaped like a white ball.
- Oxalis is a weed with trifoliate, obverse-heart-shaped leaves on long green or dark purple petioles. The flowers consist of 5 white or yellow petals with a lemon center.
- Acrid buttercup (Night Blindness) is a perennial plant with pentagonal openwork leaves, yellow flowers, bright with a glossy tint.
- Green moss (Bryochites). Most often, indicators of acidic soil are weeds of the Bryophyte department - Sphagnum, Dikran, Hylocomium.
- Cornflower is an annual plant from the Asteraceae family. Flowers blue, lilac yellow.
- Heather is a low shrub with small flowers collected in inflorescences, small triangular leaves.
Also indicators of acidic soil are weeds: lycopodium, violet, cottonwood vaginalis, blueberry, knotweed, plantain, Erica, oxalis. And also meadow grass, antennaria, lingonberry, lupine, bear's ear, ground reed grass, woodlice, and spurge. It is impossible to talk about acidic soil based on one growing weed species. To accurately determine, there must be clusters of one or two types of weeds in the garden.
What soil acidity do weeds like?
- They prefer to grow in an acidic environment: sedge, fireweed, fern, plantain, horse sorrel, horsetail, wild rosemary, mint, heather, cornflower, cinquefoil, tricolor violet, dandelion, clover, chamomile.
- The neutral environment attracts adonis, thistle, field bindweed, nettle, quinoa, red clover, and shepherd's purse.
- Chicory, spotted spurge, thyme, sage, bergenia, thistle, and mustard grow in alkaline soil.
- If nettles grow in the garden, this means that the soil contains a large amount of nutritious organic elements.
Knowing how to independently determine the acidity of the soil, you can help the plants grow painlessly in your area.
What does soil acidity affect?
Soil acidity affects the solubility, availability and uptake of nutrients by plants. Thus, on moderately acidic and acidic soils, phosphorus, iron, manganese, zinc, boron and other elements are more accessible and better absorbed by some plants. If the acidity is increased (pH = 3.5-4.0), then instead of even greater absorption of nutrients, the growth of roots and the activity of their work will be inhibited; the plants become sick from a lack of supply of necessary nutrients to the organs.
In strongly acidic soils, the content of aluminum increases, which prevents the entry of phosphorus, potassium, magnesium, and calcium into plants. Substances that negatively affect beneficial microflora begin to accumulate in the soil. The processes of processing organic matter into humic substances and then into mineral compounds available to plants will practically cease.
The alkaline environment also significantly affects many biological processes. Prevents the absorption of some macro- and microelements needed by plants. Phosphorus, magnesium, boron and zinc become unavailable to plants. In some plants, the opposite effect is observed: in an alkaline environment, the root system of plants intensively absorbs applied mineral fertilizers, even to the point of toxicity.
The optimal limits of soil acidity for various crops, ornamental parks and flowering plants were experimentally determined in agrochemical studies (Table 2). For vegetable crops, the most favorable soil acidity is neutral or slightly acidic (pH = 6.0-7.0).
Table 2. Optimal soil acidity level for garden crops in the country
| Soil pH | Name of crops |
| 5,0 – 6,0 | watermelon, potatoes, pumpkin, parsnips, sorrel |
| 5,5 – 7,0 | tomato, white cabbage, carrots, corn, garlic, cucumber, pepper, parsnip, rhubarb, beets, peas |
| 6,0 – 7,0 | lettuce, onions, legumes, pumpkin, spinach, beans, eggplants, garlic, kale, Brussels sprouts, radishes, zucchini, beets, carrots, chard, turnips, tomatoes, chives, shallots, leeks, honeydew melon, chicory, cucumbers, horseradish, spinach, rhubarb |
| 7,0 – 7,8. | cauliflower, artichoke, celery, lettuce, onion, asparagus, parsley |
| 4,0 – 5,0 | heather, hydrangea, Erica |
| 5,0 – 5,6 | juniper |
| 5,0 – 6,0 | pine |
| 6,0 – 7,0. | 1 – ornamental woody, ornamental herbaceous perennials and annuals, lawn grasses 2 – fruit crops (plum, cherry) |
| 5,5 – 7,0 | apple tree, strawberry, pear. |
| 7,0 – 7,8 | clematis |
| 4,0 – 5,0 | blueberries, cranberries, currants, gooseberries, raspberries |
| 5,0 – 6,0 | lily, phlox |
| 5,5 – 7,0 | carnation, iris, rose |
| 7,0 – 7,8 | peony, delphinium |
Types of acidity
Soils can be slightly acidic, neutrally acidic, slightly alkaline and moderately acidic. All these types of acidity differ not only in composition, but also in what can be grown in such soil.
- In medium acidic conditions you can grow: potatoes, sunflowers, sorrel, melon, corn, strawberries, hydrangea, gooseberries, cherries, apple trees.
- In slightly acidic conditions they grow: roses and lilies.
- In neutral: most garden plants: chicory, apricot, grapes, black currants, lilacs, chrysanthemums, crocuses.
- In slightly alkaline: carrots, onions, cabbage and cauliflower, parsley, asparagus, celery, artichoke, tulips.
There are other categories of acidity:
- Actual acidity is, as a rule, podzolic soils in forest areas, as well as various types of peat bogs.
- Potential soil acidity is the acidity of hardened soil; it is determined per 100 grams of dried soil and the presence of cations in it.
- Metabolic acidity - it is affected by hydrogen and aluminum, which come into chemical contact with salts. This type of acidity occurs in soils rich in humus.
- Hydrolytic is alkaline acidity and consists in the interaction of ions with hydraulic alkaline salts; this acidity is determined by the saturation of the bases.
Soil pH levels - norms for plants
The acidity scale is expressed in units from 1 to 14:
Both highly acidic and highly alkaline soils are an unfavorable environment for growing vegetables. In both cases, the absorption of nutrients is disrupted and the plants die.
What to do if the soil in the garden is acidic? If the pH is not always low, then periodic addition of alkaline substances in accordance with the initial acidity level is sufficient.
Video: How to deoxidize the soil and fertilize it at the same time
It is worse when the site is located in a lowland and there is always high humidity in the garden. Here you will have to create and maintain a slightly acidic environment yourself, which of course takes a lot of time: alkalization takes several years before it gives positive results. This could be two or three years.
The composition also affects the pH - peat soils are often acidic, loams are alkaline, chernozems are neutral, that is, the amount of humus directly affects the acid-base balance.
What does soil acidity affect?
Before choosing plants for planting on a site, it is necessary to take into account the degree of their tolerance to acidic composition. It is difficult to obtain a harvest from a planting plot that does not meet biological needs. The nutrition of unadapted species is disrupted, the digestibility of microelements is unsatisfactory, and nitrogen starvation is observed in crops. The manifestation of negative factors increases in the rainy and cold season.
In an acidic environment, iron and aluminum turn into salts; in this composition, phosphorus, calcium, magnesium and potassium are not absorbed by plants. The growing season is weak and yields drop significantly. The root system stops branching, the supply of nutrients and water to the ground part is reduced. The culture weakens and is more susceptible to infection than a strong plant adapted to acidic soils.
Acidic soils are prone to waterlogging, and pathogenic microorganisms that are dangerous to crops develop in them. In this composition, some of the nutrients settle, and carbohydrate and protein metabolism is disrupted during the growing season. The green mass loses turgor, photosynthesis is disrupted, and the color becomes pale.
The cause of increased acidity in the soil may be:
- oversaturation with randomly applied fertilizers of one type;
- natural factors, for example, peatlands;
- non-compliance with crop rotation;
- influence of nearby chemical plants.
A change in acid level is indicated by the appearance of new weeds in the area that can only grow on this composition.
Why is acidic soil dangerous?
There are more acidic soils in our country than alkaline ones, especially in the middle zone. Alas, although some plants thrive on them, for most, acidic soils are dangerous, since the water, carbon, protein and nitrogen balance of the soil is disturbed, which entails many problems.
When snow melts, moisture is poorly absorbed, and a crust quickly forms on the surface, which prevents the penetration of not only water, but also air into the soil (that is, the earth “does not breathe”), as a result of which some of the plants unadapted to these characteristics die, and the remaining ones grow weak and produce very little yield.
Lack of many micro- and macroelements needed by plants (for example, nitrogen, calcium, magnesium, sulfur, phosphorus). Firstly, there are fewer of them in acidic soil than in neutral or alkaline soil, and secondly, even with abundant application of fertilizers, these elements are quickly converted into a form that is poorly absorbed by plants.
Increased content of iron, copper and zinc leads to inhibition of plant growth, and in saline soils it can sharply increase soil toxicity. Transparent watery spots appear on the lower leaves and the leaves soon fall off.
Beneficial bacteria that improve the structure and cleanse soils with lower acidity do not survive here, but pathogenic microflora develop actively, so many acidic soils are often “sick”, infecting plants.
The presence of toxic substances that are not washed out or neutralized naturally in acidic soil inhibits the development of the root system and leads to plant diseases. Thus, heavy metals quickly accumulate in acidic soils, which then penetrate into plant tissue. A number of elements that are generally beneficial for plants (aluminum, iron, manganese) form toxic compounds in acidic soils and harm plants.
What fertilizers deoxidize the soil?
If acidic soil prevents garden plants from growing, what should you do first? Firstly, the indicators may differ depending on what crops grew in the garden before, so you need to check each one and apply deoxidizers only where the indicators are lower.
Secondly, pay attention to what fertilizers are available for acidic soil - perhaps it’s enough to just feed the soil with wood ash or compost, and it will recover.
If there is a lot of acid, then you will have to use solid fertilizers, such as chalk, quicklime, dolomite flour.
Chalk
Chalk is a substance containing calcium. To make it work faster, you need to make sure that the size of the grains does not exceed 1 mm in diameter. For particularly acidic areas, 300 g per square meter will be required, for medium acidity - 200 g, for slightly acidic areas - 100 g.
The chalk is evenly distributed over the area, leveled with a rake, and then dug up. It's better to do this in the fall. Next year, the chemical composition of the soil will partially change and it will be possible to plant vegetables in the garden bed.
Slaked lime
It is recommended to carry out liming in several stages, adding 1/3 of the required amount annually for three years. For example, if you need to add 5 - 7 kg per square meter with very strong acidification, then in the first year they lay and dig 2 - 3 kg, in the second and third 1.5 kg of lime.
For moderately acidic soil - 4 - 5 kg per square meter, for slightly acidic soil - 2 kg. The depth should not be less than 20 cm from the surface. Before use, the lime is poured with water and left for a while.
To remove weeds, you can pour more of the substance where plantations of plantain or other acid-loving plants grow.
Wood ash
Wood ash is suitable for medium and slightly acidic soils. If the degree is very strong, you will have to apply a lot and wait a long time for the earth to recover. In addition, with this method there is no accumulation of calcium in the soil, and almost all vegetable crops need it.
The fact is that all calcium is used to reduce acidic compounds. Calcium is necessary for the prevention of various rots, especially vertex rot in tomatoes and peppers.
Experts recommend using ash together with other substances - chalk or lime, as well as dolomite flour. Per square meter you will need 200 g of wood ash infused with water. If you use peat ash, then the amount should be 2 times larger.
Compost
Suitable for neutralizing slightly acidic soil and maintaining optimal levels of substances in the soil. Compost itself has a neutral reaction. It is not suitable for heavily acidified areas.
Compost can be produced independently from vegetation debris, fallen leaves, manure, hay or dry straw as a carbon component. It takes a long time to wait for the substance to mature. Usually this takes a year and a half. The advantage of compost is that it restores soil microflora well and promotes the accumulation of humus in the soil.
To maintain a slightly acidic soil reaction, it is necessary to apply 10 kg of compost per square meter annually.
Dolomite flour
Limestone flour or dolomite is the safest and most environmentally friendly way to restore soil when compared with the use of lime. In addition, nutrients such as magnesium and calcium in the form of oxides also enter the soil.
With strong acidification, 600 g of dolomite flour will be required, with average - 500 g per square meter, with weak - 350 g.
If you dig up the soil after applying fertilizer, the effect will appear faster. If you simply scatter it around the area, the substance will penetrate into the soil only after a year. You need to make sure that the flour is finely ground - this way it will work faster.
Green manure
Green manure not only contributes to the deoxidation of the soil, but also nourishes it with various microelements and nitrogen. This is a completely environmentally friendly way to fertilize acidic soil. True, you will have to spend 2–3 years on it. If the pH is very low, it is recommended to first add dolomite flour, spruce or lime, and then plant green manure in the second or third year.
Another advantage of green manure plants is that they facilitate the penetration of air and water into the soil. After the roots rot, tubules remain in the soil through which moisture and oxygen enter. At the same time, the soil becomes loose, and beneficial bacteria begin to multiply in it.
It is most profitable to sow green manure to prevent acidification before winter, after harvesting. They have time to grow before frost, but do not have time to set seeds, so their growth is easy to control. It is not recommended to dig up green manure with soil before winter - this washes away nutrients and takes a lot of effort. If you plant in the spring, then after a while the crops are cut and dug up so that they begin to decompose faster.
Depending on what crop you plan to grow in the garden, you need to choose a suitable green manure plant. For example, cruciferous mustard is not planted before cabbage, otherwise the latter will start to hurt, and after vetch it is better not to grow beans or peas. The most useful crops in this regard are lupine, clover, vetch, peas, phacelia, sweet clover, white mustard and cereals - oats or rye.
How to reduce acidity
Acidity can be reduced by adding fluff lime and dolomite flour. Chalk or wood ash will also work.
Soil liming
To carry out liming, it is better to know more accurate indicators of the soil environment in order to correctly calculate the rates and methods of applying lime or other deoxidizers. After all, the wrong dosage can harm cultivated plants.
- With an acidity of 4.0 to 5.5, you need 2 kg of dolomite flour per 1 m². Apply it in the fall for 3 years, checking the instructions on the package.
- Wood ash under the same conditions needs up to 500 g per 1 m².
Photo: Soil liming
Liming is best done in the fall, once every 4-5 years. However, you don’t need to liming thoughtlessly, just because your neighbors did it or your friends recommended it. After all, the acidity of the soil can vary greatly throughout the entire site and even in neighboring beds.
And even if you have no complaints about your six hundred square meters, periodically check the soil acidity level to be on alert and take the necessary measures in time.
Soil acidity and pH indicators
Soil acidity or pH is a biochemical indicator that characterizes its ability to exhibit (neutralize) the properties of acids. During the exchange of hydrogen ions with soil minerals and organic matter, acids and bases (alkalis) are formed in the fertile layer. pH indicates their balance in the soil solution; it is designated by numbers from 1 to 14. The lower the pH number, the more acidic the environment. What determines soil acidity?
- The determining factor is the original material from which the soils are formed: on sandstone, granite - more acidic, on limestone - alkaline.
- A gradual increase in acidity occurs in regions with frequent heavy rainfall. Moisture, accumulating in the soil, washes minerals and salts from the root layer.
- Leaching may be caused by intensive watering with low pH (acidic) water.
- Acidification occurs when excessive application of plant residues, organic and mineral fertilizers to the soil.
- Poor air permeability of the soil contributes to an increase in acidity. If organic matter decomposes without access to oxygen, organic acids and carbon dioxide released as a result of a chemical reaction remain in the soil.
Interesting! In the Russian Federation, approximately a third of agricultural land is acidic and requires regular liming. This is most of the soddy-podzolic, soddy and gray forest soils of the middle zone and Siberia. In Western Europe there are almost 60% of such lands.
Let's consider the optimal soil acidity indicators for plants, and below in the table we specify them in the context of garden and vegetable crops.
The most acceptable acidity level for most cultivated plants is in the range from 5.5 to 7.5 - these are slightly acidic (5-6), neutral (6.5-7) and slightly alkaline (7-8) soils. A pH below 5 means a medium to strongly acidic reaction, above 8 means an alkaline reaction. An acid-base balance above 9 indicates that we have saline-carbonate soils or even saline soils.
Optimal acidity range for common horticultural crops
| Garden crops | Horticultural crops | ||
| Plant | pH range | Plant | pH range |
| Potato | 5,0–5,5 | Raspberries | 5,0–6,0 |
| Onion | 6,4–7,9 | Strawberry | 4,5–5,5 |
| Carrot | 5,5–7,0 | Apple tree | 6,0 – 6,5 |
| Beet | 6,8–7,5 | Pear | 6,2–6,7 |
| Cabbage | 6,7–7,4 | Currant | 6,0–7,4 |
| Peas | 6,0–7,0 | Sea buckthorn | 6,0–7,4 |
| Garlic | 6,0–7,0 | Chubushnik | 6,5–7,0 |
| cucumbers | 6,0–7,9 | Lilac | 6,5–7,0 |
| Tomatoes | 6,3–6,7 | Forsythia | 3,5–4,0 |
| Pepper | 6,3–6,7 | Rhododendron | 4,0–4,5 |
| Eggplant | 6,3–6,7 | Cowberry | 4,0–4,5 |
| Zucchini | 6,0–7,0 | Cherries | 6,7–7,5 |
How to determine the acidity of the soil on the site
The indicator of soil acidity level is indicated by pH.
It is ranked as follows:
- strongly acidic soil - if the indicator is less than 4.0;
- medium acidic soil – pH from 4.0 to 5.5;
- slightly acidic – pH from 5.5 to 6.5;
- neutral – pH from 6.5 to 7.5;
- slightly alkaline – pH from 7.5 to 8.0;
- medium alkaline – pH from 8.0 to 8.5;
- highly alkaline – pH more than 8.5.
Finding out what the acidity of your soil is is extremely simple. Buy litmus papers with a scale, dilute a handful of soil in a glass of water, strain the sediment and dip the paper into the solution - it will change color. Compare it with the color of the scale and find out the level of soil acidity. Of course, you can only be told as accurately as possible about the acidity of the soil in a laboratory.
The most effective and reliable way is to contact a specialized agrochemical laboratory or similar organizations. This is the most accurate and least labor-intensive method, which, however, requires some material costs.
You can reliably and simply determine the acidity of the soil yourself using special instruments and objects.
Photo: Checking acidity with special devices
Litmus paper for determining acidity
Litmus paper consists of a number of thin strips impregnated with a special composition. They change their color depending on the acidity of the soil in the area under study. You can buy such an indicator in a specialized store, pharmacy, or in stores for summer residents. The soil for analysis using litmus paper must be taken in several places at a depth of about 30 cm - this is where the main layer of roots is located. If you are determining the pH for several beds, then take samples from each separately. To establish an average for the entire site, samples are taken in several different locations distant from each other. The selected soil should be poured into a two- or three-layer gauze bag or simply carefully wrapped in gauze. Then place the sample taken into a container with clean, free of foreign impurities, spring, bottled, and best of all, distilled water. There should be 5 times more water than land. After thorough shaking, after 10 minutes, lower the litmus paper into the water. By the color it acquires, you can easily determine whether you need to lime the soil - you just need to compare it with the scale included in the instructions.
Photo: Testing acidity using litmus paper
pH soil acidity meter
This device combines several functions and helps determine, in addition to acidity, also humidity, temperature, density and illumination of the soil. I would like to recommend purchasing such a device to all summer residents. It is inexpensive, easy to use, gives the desired result accurately and quickly, and will serve you for many years.
The importance of soil acidity for growing plants
When it comes to soil acidity, an inexperienced gardener will think that the interlocutor is trying to show his know-it-all.
As if flaunting abstruse terms, using as an argument a completely incomprehensible letter combination pH. And at the same time, pH in the abbreviated Latin transcription literally means “weight of hydrogen.” It characterizes the quantitative composition of hydrogen ions in the medium and is called acidity.
In agronomy and horticulture, information about it is extremely important. Its influence on the process of assimilation of vital substances by green crops and, as a consequence, further vegetation, has been deeply studied and is beyond doubt.
In soil with optimal acidity, manganese, boron, zinc, and iron are well broken down. But a shift in this indicator towards increased acidity or, conversely, alkali, immediately affects the process of plant development.
Here it is appropriate to give an example of heartburn in humans. As soon as a malfunction occurs due to certain circumstances related to food intake, this immediately negatively affects the functioning of the stomach and well-being in general. Similar processes occurring in nature are similar to those in humans.
It cannot be unequivocally stated that for agricultural or garden crops, a high or low pH is good or bad. Each natural individual “wants” to be in its own comfortable acid-base conditions.
However, most of them prefer slightly acidic or neutral soil characteristics.
Table of optimal soil acidity (pH) for various plant crops
What the numbers after the abbreviation pH mean, which is found on packages (bags) of soil mixture (planting material) sold in specialized stores, not even every experienced summer resident will be able to explain exactly.
And if you ask what the acidity of the soil is in his area, only a few will answer. But it is precisely this characteristic of the soil that largely affects the yield of a particular garden crop. It is not for nothing that there are special tables that indicate recommended acidity values for various plants. The author considered it correct to place several tables in the article, although some of them repeat each other. But if you look closely, you can find differences. In addition, this gives the reader more opportunities to determine the optimal soil pH for the plant he is interested in, since none of the tables contains an exhaustive list of all garden crops.
What do the numbers after the pH symbols mean? Indeed, many recommendations for growing plants do not indicate these, but the characteristics of the soil - medium, strongly acidic, and so on. These tables (somewhat duplicating each other) explain everything well.
Why do you need to take into account the pH of the soil on your site? Each garden plant, during its development, consumes a certain set of microelements (nutrients) from the soil. Their growth and further fruiting depend on how saturated the earth is with them.
That is why good owners always not only adhere to the rules of crop rotation, but also artificially regulate the acidity of the soil. If not on the entire site, then on certain segments of it - for sure. Removing a layer of soil, delivering fertile soil to the territory - no summer resident will seriously consider such a technology, given the complexity of implementation, labor costs and high cost. But many garden crops are planted in pre-prepared holes. For example, berry bushes, potatoes.
Knowing what acidity the soil needs for a particular plant, it is enough to load the soil mixture you made yourself into these holes. Alternatively, fertilize the soil with appropriate fertilizer. With such a competent approach, you can count on high yields even on land that is not entirely suitable for gardening.
How to quickly determine pH
Farmers take soil samples to the laboratory or purchase a pH meter - a device for measuring the level of acids that appear in the soil solution. But this method is extremely inconvenient due to the need to dissolve handfuls of soil in distilled water, and remove soil samples from a depth of 6 cm. In addition, you need to check the soil in the garden in different places, taking samples 5 times at intervals of up to 30 cm.
Since you need to check the acidity of the soil at home quickly and without unnecessary hassles , gardeners use acmus strips, phenolphthalein and methyl orange . These test substances change color in an acidic environment.
It is important to know. All types and varieties of cabbage, beets, garlic and onions feel comfortable in neutral soils. Zucchini, cucumbers, eggplants, peas and potatoes prefer slightly acidic areas. An acidic environment is ideal for pumpkins, carrots and tomatoes.
Ordinary table vinegar will help to carry out a test , for which a handful of earth (1 tsp) is poured with a few drops of liquid on the glass. Bubbles and hissing will appear upon contact with an alkaline medium; if they do not exist, then it is acidic.
You can use red cabbage for the dough. Then the soil solution is filtered using distilled water. Squeeze the juice out of the cabbage and add a few drops of alcohol to it. The solution and cabbage-alcohol liquid are combined. If the color of the tester becomes more scarlet, the soil is acidic; if it turns blue or purple, the substrate is alkaline.
10 blackcurrant leaves you can determine the environment of the earth . In an acidic environment, the infusion will turn red, in a neutral environment it will turn bluish, and in a slightly acidic environment it will turn green.
Weeds also respond to pH, so they can be used to judge which environment is more comfortable for them. Namely:
- in an acidic environment there will be a lot of growth of nettle , creeping buttercup, heather, plantain, common and horse sorrel, pike and pickerel, white and sorrel, buttercup, popovnik, sphagnum and green mosses;
- in a slightly acidic environment you will have to fight with clover, field birch, coltsfoot, odorless chamomile, plantain, dog violet and meadow cornflower;
- a neutral environment is suitable for field bindweed, spring Adonis (adonis), white clover, and sow thistle;
- alkaline - a comfortable environment for white dormancy, field mustard, larkspur and poppy .
Consider the table of soil acidity for plants ( table ):
| 5,3-6,0 | For acidophiles | For plants growing in acidic soil |
| 6,0-7,2 | For neutrophils | For plants that prefer a neutral environment |
| 7,3-8,1 | For basiphiles | For plants that prefer an alkaline environment |
A neutral or slightly alkaline reaction (pH 6.6-7.0) in the soil is suitable for plants: white cabbage and other varieties: parsnips, onions, peppers, beets, celery, asparagus.
A slightly acidic reaction (pH 6.3-6.7) is suitable for plants: beans and eggplants, peas, melons and zucchini, kale, potatoes, lagenaria and loofah, cucumbers, lettuce and beans, spinach. Neutral or slightly acidic soil is loved by garden flowers : roses, primroses, gillyflowers and chrysanthemums.
What plants love acidic soil (list):
- vegetables - potatoes, carrots, radishes, turnips, tomatoes, pumpkin, sorrel;
- berries – lingonberries, blueberries, strawberries, cranberries;
- herbs and flowers - azalea, evergreen erica, heather and hydrangea, rhododendrons and ferns, chicory and conifers.
pH determination
Special pH meter
Thanks to a special device, you can obtain information more accurately and it will be more reliable. However, the method is not convenient: you can dissolve the soil only in dechlorinated water and take the soil at a depth of 6 centimeters. You need to check the result five more times in different places at a distance of thirty centimeters.
You can determine the acidity of the soil without resorting to the services of specialists. Buy litmus paper at your nearest pharmacy. Litmus packages usually have a small acidity scale on them. Place some soil in a thick cloth. Make several of these bags.
Immerse them in salt-free water (distilled or rainwater) for five minutes in a 1:1 ratio. After this, dip the litmus into the liquid for one or two seconds and quickly pull it out or drop it onto it. Compare the color with the scale on the packaging or from the Internet.
Experiment with vinegar
Add a few drops of vinegar to a small amount of fresh soil.
- Foam formation - alkaline environment;
- Formation of a small amount of foam - neutral environment;
- Lack of foam means the environment is acidic.
Read also: Rambutan fruit: description, beneficial properties and features of growing at home
Red cabbage
Grind the leaves and squeeze the juice out of them, add a little alcohol. For the test, use only a filtered soil solution with dechlorinated water. Redness of the tester indicates that the soil is acidic. Its blue color indicates an alkaline environment.
Black currant leaves
Add about nine currant leaves to half a liter of boiling water. The liquid is allowed to cool, after which a little fresh soil is added and stirred.
- Blue color is a neutral environment;
- Green color - slightly acidic environment;
- Red color means acidic environment.
External signs
Sometimes you can determine high acidity by the following signs:
- A whitish tint is a distinctive feature of acidic podzolic soils;
- Weeds such as white grass, creeping buttercup, field grass and the like grow well;
- Abundance of moss;
- Wheat does not overwinter;
- Clover and alfalfa grow poorly.
Acidity measurement
Soil acidity can be determined in several ways:
- Using strips of indicator paper. Paper can be purchased at any specialty store. In order to use this paper, you need to dig a hole 30 cm deep. The soil from the walls of this hole must be scraped off in at least 4 places. Then mix the soil at the bottom of the hole and moisten it with water, crumple up the indicator paper and place it in the wet soil. The color of the paper will change depending on the acidity: red is strong acidity, pink is medium acidity and yellow is weak acidity. If there is no acidity, the paper will turn green.
- The acidity level can also be determined by external signs. If the water on the surface of the earth (in puddles) becomes red in color with a rainbow-colored tinge, this means that the soil is highly oxidized.
- Acidity can also be determined by the weeds growing on the soil. The following usually grow on acidic soil: horsetail, cornflowers, plantains, buttercups, fireweed, mint, violets, daisies, sedge and heather. Nettle, chamomile and quinoa usually grow in slightly acidic soil.
- There are also several other ways that will indicate the acidity of the soil: to do this, place a small piece of earth on the glass and moisten it with vinegar. If the soil foams, it means that it is very acidic; if there is no reaction, then the acidity is normal.
- You can also determine acidity by the color of the beet tops. If the tops are reddish in color, then the soil is acidic, if the leaves are green, then the acidity is normal, and if the beets have a red petiole, then there is no acidity at all.
- Soil acidity can be roughly estimated using simple pH meters.
Sources used:
- https://flowery-blog.ru/interesnoe-o-cvetah/rasteniya-lyubyashhie-kisluyu-pochvu.html
- https://udobreniya.net/kislaya-pochva-chto-delat/
- https://www.botanichka.ru/article/kislotnost-pochvyi-kak-opredelit-i-raskislit/
- https://agrognom.ru/weeds/kak-opredelit-kislotnost-pochvy-po-sornyakam.html
- https://sad6sotok.ru/%d0%ba%d0%b8%d1%81%d0%bb%d0%be%d1%82%d0%bd%d0%be%d1%81%d1%82%d1 %8c-%d0%bf%d0%be%d1%87%d0%b2%d1%8b.html
- https://golosros.ru/sornyaki/kak-opredelit-kislotnost-pochvy-po-sornyakam-foto-trav-indikatorov-kisloty
- https://mirogorodov.ru/kislotnost-pochvy.html
- https://1decor.org/rasteniya/udobreniya/kislotnost-pochvy.html
- https://sadim.guru/kislotnost-pochvy-vazhnost-ph-grunta-dlja-rastenij/
Determining soil acidity using acids
While I was writing the text, I started thinking. We studied something about soil acidity at school, in natural history classes. They even carried out experiments. Fortunately, I found a detailed description of a method by which you can roughly determine whether the soil on your site is acidic or alkaline.
Using vinegar
The method, which can be used at home, is based on the chemical reaction of vinegar with minerals. To do a test test yourself, take a handful of soil from a depth of about 20 cm. Spread in an even layer on a plate or board and pour in vinegar. The result will not be long in coming.
If bubbles appear on the surface, the mud slurry bubbles slightly and a quiet hiss is heard - the soil is neutral or alkaline. The vinegar reacts with the limestone, which you can observe in the analysis.
No changes occur - the soil is acidic. If in doubt, mix a handful of earth with water and add soda. The paste will begin to bubble and sizzle.
The method is quite primitive; it can be used at home for preliminary analysis. To find out the exact pH level, methods with vinegar or soda are not suitable.
Using grape juice
Use natural grape juice. Store-bought has virtually no natural ingredients and is diluted with water. There will be a reaction, but it will be so weak that you may not notice anything.
To understand whether the soil in your area is acidic or alkaline, collect 50 ml of juice in a glass or other transparent container. Place a small lump of earth and watch the reaction.
If there are no changes, the soil is acidic. If bubbles appear, light foam appears, and the juice changes color, the soil is neutral or alkaline.
The method is also not accurate and is suitable, for example, when searching for a suitable plot for a garden or vegetable garden. If you determine that the soil is acidic, think about it. Maybe look for another option for the garden and not spend money on liming?
Hydrochloric acid
Another interesting method that gardeners use when planting a garden. It can be used to determine whether the soil is alkaline. It is also easy to determine the presence of lime in the soil.
Dig a hole one meter deep. Carefully, in a thin stream, pour 5% hydrochloric acid along the vertical wall. At a depth of 50-60 cm from the surface, the acid will react with lime if it is present in the soil. You will notice a "boiling" and hear a slight hiss.
This is fine. It is worse if such reactions are observed at a higher level. Trees in such a location will turn yellow prematurely, which will worsen the yield. The absence of any reactions indicates acidic soil.