Category: Mineral fertilizers Reading time: 9 min · Views: 1,160
A nutrient such as phosphorus cannot be replaced by any other. It, along with nitrogen and potassium, is responsible for the viability of the crop and the flow of all metabolic processes inside the plant. They learned to solve the problem of phosphorus deficiency a long time ago, using special enriched phosphorus fertilizers.
Phosphorus and its role in plant development
Phosphorus is needed by plants at all stages of the growing season:
- This element is part of the nucleic acids responsible for the transmission of hereditary information. Therefore, a high concentration of phosphorus is observed in pollen and seed embryos.
- Normal seed germination, the formation of a good root and nutrition in the initial phases of ontogenesis depend on the supply of phosphorus to the embryo. The deficiency of this element in this period cannot be corrected by later application of fertilizers.
- In young plants, phosphorus is evenly distributed throughout all parts and organs. By combining with proteins and forming phosphoproteins, it participates in many biochemical reactions, sugar synthesis, cellular respiration, etc. By combining with lipids, it forms phospholipids necessary for the construction of cell membranes.
- As the plant matures, phosphorus moves to the generative organs. During this period, it is necessary for the formation of full-fledged flowers, productive pollen, and for the formation of ovaries and seeds. A low supply of this element leads to inhibition of flowering, ovary formation and ripening.
This element is responsible for the accumulation of nutrients in roots and wood, therefore it is important for good wintering of perennial crops. By influencing root formation, it increases resistance to summer droughts. Such a variety of functions of phosphorus makes monitoring its content in the soil an important task for the grower.
The lack of phosphorus can be judged by the state of the green parts of plants. Additionally, this problem is signaled by scanty and delayed flowering, abscission of ovaries, a decrease in the sugar content of fruits and roots, poor starchy and watery tubers, and a decrease in drought and frost resistance of plants.
The importance of phosphorus for plants
To understand why phosphorus fertilizers are needed, it is necessary to find out the effect of P on representatives of the plant world. The substance is a basic component of nutrition, as it is part of DNA and RNA. It is responsible for the generative function of plants, therefore, due to phosphorus deficiency, vegetation can die completely.
Despite the fact that nitrogen and potassium are important for plants along with phosphorus, the absence of the latter in the soil can cause the death of the planet due to the total depletion of the earth's flora.
Signs of element deficiency
Phosphorus deficiency can be determined without resorting to laboratory soil analysis. When it is deficient in plants, the following manifestations are observed:
- acquisition of foliage of a dark green color, changing to purple;
- change in the shape of leaf plates and premature falling of flowers;
- the appearance of spots with a darker shade on the back of the foliage;
- crushing of crops;
- loss of the stem from the soil due to underdevelopment of the root system.
Timely use of phosphorites will help prevent such problems, ensure abundant flowering, preserve the harvest, and decorativeness.
Supply of Russian soils with phosphorus
In general, Russian soils are well supplied with phosphorus. But here there is the following problem: the difference between gross and mobile phosphorus.
Phosphorus compounds in soil can be contained in different forms - phosphate, hydrophosphate and dihydrogen phosphate. Gross phosphorus is an indicator of the concentration of a given element in all forms. But their availability to plants varies significantly. If dihydro- and hydrophosphates are mobile and well absorbed by plants, then phosphates are the opposite. They are poorly soluble and practically unavailable for nutrition.
It follows that the content of gross phosphorus does not give an objective idea of soil fertility. On most Russian soils, obtaining satisfactory yields is impossible without additional supply of available phosphorus.
The connection between phosphorus and soil fertility? (click to expand)
Important! The supply of phosphorus correlates with the level of soil humus. Analyzes show that there is more of this element in the upper, high-humus layers of chernozems than in the lower ones (see → where to get chernozem). Therefore, when feeding plants with phosphorus fertilizers, we must not forget about the simultaneous addition of organic matter.
Signs and causes of phosphorus deficiency
Deficiency of the chemical substance has a detrimental effect on crop growth, generative functions, and fruiting. In order to timely balance the soil composition, providing adequate nutrition to the plant, it is necessary to monitor its condition.
Signs of phosphorus deficiency:
- change in the color of the shoots to dark green and then to crimson-violet;
- leaf deformation and falling;
- formation of necrotic spots with characteristic darkening;
- underdevelopment of the root system, which is why the plant “falls out” of the soil;
- growth retardation.
If plantings suffer from a lack of phosphorus, an accurate determination of what is causing the starvation will help eliminate the problem. Main reasons:
- The concentration of a chemical compound in the soil-retaining complex (it becomes difficult to reach).
- Violation of the rules for laying phosphorus-potassium fertilizers.
- The use of intensive farming technology, which led to the poor performance of soil microflora.
- Removal of phosphorus by cultivated crops without subsequent enrichment of reserves.
- Inorganic type of soil cultivation.
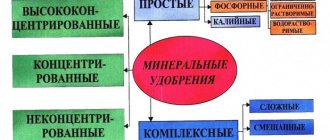
General classification of phosphate fertilizers
Like all other types of fertilizers, phosphorus fertilizers are divided by origin into organic and mineral:
| Organic phosphorus-containing fertilizers | Mineral phosphorus-containing fertilizers |
|
|
In mineral fertilizers the concentration of total phosphorus is much higher than in organic fertilizers. However, they differ greatly from each other in the bioavailability of this element.
When choosing a mineral phosphorus fertilizer, you have to take into account not only the percentage of phosphorus indicated on the packaging. The formula of the phosphorus compound is more important. Substances that contain the H2PO4 ion in the formula are absorbed by plants much better than those that contain only the acidic residue PO4.
The importance of phosphorus-potassium fertilizers
Versatility is an important characteristic of agrochemicals. This is exactly what phosphorus-potassium complexes have, which has contributed to their growing popularity among farmers and gardeners. They are applied both in autumn and spring. The use is not limited during the growing season. It is only necessary to take into account the consumption rate, which depends on the phase of crop development.
Common fertilizers from this series are nitrophoska and nitroammophoska. Externally, it is a granular gray powder with a slight pinkish tint. Thanks to the special formula, plants absorb the applied products without leaving any residue. Not a single gardener can do without the use of tuk when planting trees, as well as a florist who grows potted plants.
For your information!
Phosphorus and potassium, when simultaneously exposed in different directions, enhance seed germination, strengthen flower buds and fruits, increase the content of sugars and carbons in root crops, and increase productivity.
Water-soluble phosphorus fertilizers: description and application
All phosphorus-containing mineral fertilizers are divided into water-soluble, sparingly soluble and insoluble (see → types of mineral fertilizers). The group of water-soluble superphosphates includes:
- Simple superphosphate. Contains 16-20% phosphorus in the form of dihydroorthophosphate and phosphoric acid. The composition also contains calcium, sulfur and traces of magnesium. Find out → instructions for using superphosphate, application rates and watering.
- Double superphosphate. The composition is similar to simple superphosphate, but the concentration of phosphorus is doubled - up to 46-49%. Find out → the use of double superphosphate as a fertilizer + reviews.
Both substances are produced in granular form, easily form aqueous solutions, and in terms of phosphorus digestibility they are the best of all types of phosphorus fertilizers.
Superphosphates are used for basic soil application in spring and autumn, as well as for summer root feeding with solutions:
| Type of superphosphate | Norm for application to soil | Norm for liquid fertilizers |
| Simple | 50-70 g per 1 m2 in open ground 80-100 g per 1 m2 in greenhouses 400 g per seedling when planting fruit crops 60 g per 1 m2 in the trunk circles of mature trees 3 g per hole when planting potatoes. | 2 tablespoons per 10 liters of water |
| Double | 20-30 g per 1 m2 in open ground 20-25 g per 1 m2 in greenhouses 15 g per 1 m2 in the trunk circles of mature trees 1 g per hole when planting potatoes and vegetable seedlings. | 20 g per 10 liters of water |
The solubility of superphosphates increases as water temperature increases. Therefore, it is better to prepare solutions in hot water and then cool them before watering.
To save time, concentrated master suspensions are sometimes prepared from simple superphosphate, which are then diluted to the desired concentration. To do this, take 20 tablespoons of the substance per 3 liters of hot water and dissolve with stirring until a homogeneous milky liquid is obtained. It can be stored in a closed jar. To prepare a working solution, add 150 ml of mother liquor to 10 liters of water and use it for summer feeding.
Superphosphates work well on all types of soil with any acidity. They are especially effective on depleted podzols in central Russia. In black earth regions, before using these fertilizers, you need to make sure that there is not enough available phosphorus in the soil.
Complex fertilizers and rules for their use
For garden crops and flowers, both potassium-phosphorus and nitrogen fertilizers, as well as various microelements, are important. At dachas and at home, treatments are often carried out with products containing copper and calcium.
Calcium nitrate
Calcium nitrate is a complex preparation that is used:
- in solid form for digging;
- diluted with water for root feeding and foliar spraying.
The latter is relevant for cucumbers and tomato seedlings planted in open ground. To prepare for 1 liter, it is enough to take 2 g of powder.
Copper sulfate
The drug is effective when spraying plants against various diseases and pests. However, when it gets into the soil, it binds phosphorus compounds, which can cause crops to begin to experience phosphorus deficiency. To prevent this from happening, you should strictly follow the instructions for use of the product. The main area of use is gardening and floriculture.
When growing plants, one should not forget about phosphorus. The use of nitrogen-phosphorus-potassium fertilizers will allow you to grow healthy crops that will give a rich harvest and will delight the owners for many years.
Limited soluble phosphate fertilizers: conditions of use
The second group of phosphorus fertilizers includes substances that are not able to dissolve in water. They go into solution, including soil, only under one condition - if it has an acidic reaction. Therefore, this group can only be effective on acidic soils:
- Precipitate. A light gray powder that contains from 27 to 31% phosphorus in oxide form. Used for basic application in the amount of 70 g per 1 m2.
- Thomasslag. Thermophosphate, which is a waste product from the metallurgical industry. Contains 12-20% phosphorus oxide. Used for basic application in the amount of 100 g per 1 m2.
- Renanium phosphate. Thermophosphate, very effective on acidic soils. Phosphorus oxide content – up to 34%. With the main application, a normal amount of 50-70 g per 1 m2 is required.
It is preferable to apply limitedly soluble phosphorus compounds to the soil in the fall. They pass into the soil solution slowly, so they are not effective when applied in spring.
Tip #1. Fertilizers from this group can be used to prepare liquid fertilizer for plants on saline alkaline soils. But to do this, they need to be diluted not in ordinary water, but in a solution of citric acid (2 tablespoons of acid and 30 g of fertilizer per 10 liters of water).
Signs of phosphorus deficiency
The lack of phosphorus in the soil is reflected in this way:
- the color of the plant becomes darker, gradually acquiring a purple-black tone;
- leaves change in shape and fall off;
- the lower leaves become covered with dark spots;
- plants grow as a bush, not reaching the desired height;
- productivity deteriorates;
- leaf petioles acquire a lilac hue;
- leaf internodes become shorter;
- roots grow at a slower pace.
The importance of phosphorus for plants
With the timely use of phosphorus fertilizers, it is quite possible to prevent such negative consequences.
Sparingly soluble phosphorus fertilizers: application and limitations
The least effective of phosphorus fertilizers are substances that are insoluble not only in water, but also in weakly acidic solutions. To go into solution, they need to be exposed to strong acids that are not used in plant growing - nitric or sulfuric. This group includes the following fertilizers:
- Phosphorite flour. Dark gray powder, which is prepared by simple mechanical grinding of apatites. Depending on the brand, it may have a different phosphorus oxide content - from 20 to 29%. The rate of application to the soil is 20 kg per 1 hundred square meters.
- Vivianite. A mineral extracted from peat bogs and iron ores. It is used as a fertilizer in powder form. Phosphorus oxide content -12-26%.
Restrictions for use on acidic, dry and peaty soils (click to expand)
Sparingly soluble fertilizers are used to a limited extent - on very acidic, recently drained peat-bog soils . On soils fertilized with these substances, only those crops are sown that can absorb phosphorus from sparingly soluble compounds - mustard (see → application as fertilizing), buckwheat, peas, lupine, sweet clover. Phosphorite flour gradually deoxidizes problematic soils and makes them suitable for farming.
Phosphorus in complex fertilizers
Phosphorus is a mandatory component of complex fertilizers:
| Fertilizer name | Phosphorus content | Application rate |
| Ammophos → how to apply fertilizer | 52% | 15-30 g per 1 m2 |
| Ammophoska → instructions for use | 15% | 25-30 g per 1 m2 |
| Diammofosk → how to use + reviews | 26% | 20-40 g per 1 m2 |
| Azofoska → application | 9-22% | 20-30 g per 1 m2 |
| Monopotassium phosphate | 50% | 10-15 g per 10 liters of water for irrigation |
Ash should also be considered a complex phosphorus-containing mineral fertilizer. The phosphorus content in it depends on the initial raw material burned. In wood ash - about 2.5-3%, in dung ash - 4.8%, in cereal straw ash - about 8% phosphorus.
Ready-made mineral complexes always contain more or less phosphorus. It varies depending on the purpose of the fertilizer. The highest concentration of this element is in compositions for fertilizing before flowering, during the fruiting period and for autumn fertilization of plants.
Popular phosphorus-containing fertilizers
Superphosphate
The most popular phosphorus fertilizer is double superphosphate; it is most often used in the garden. Simple and double superphosphate differ from each other not only in phosphorus concentration: simple superphosphate (16–22%), double (up to 45%). In addition, double superphosphate also contains about 15% nitrogen compounds and 6% sulfur, and, unlike simple superphosphate, it does not contain gypsum. Both are suitable for use in all areas and for any crops: flowers, vegetables, trees.
Bone meal, or phosphonitrogen
Natural fertilizer (contains 15–35% phosphorus) – a product of bone processing. In addition to phosphorus, bone meal contains calcium, biologically active substances and trace elements (magnesium, sodium, iron, copper, zinc, manganese, cobalt, iodine). Serves as a comprehensive fertilizer for vegetables (tomatoes, peppers, cucumbers) and flowers. Indoor crops - palm trees, vines, ficus - respond especially well to bone meal. In its properties, bone meal occupies an intermediate position between superphosphate and phosphate rock. Completely decomposes in the ground in 5–8 months.
Phosphorite flour
Slightly soluble phosphorus fertilizer (phosphorus - no less than 20%, calcium - no less than 34.8%) with a very long-lasting effect when applied in high doses before planting (from 100–200 to 500 g per 1 m2). They are used only for autumn digging on acidic soils or for preparing acidic composts - manure and peat: in an acidic environment, phosphorus gradually transforms into a form accessible to plants. Do not use on soils with neutral and alkaline reactions. Phosphorite flour cannot be mixed with lime fertilizers and ash; it can be mixed with ammonium sulfate, ammonium nitrate, potassium chloride and simple superphosphate.
Phosphorus content in organic fertilizers
Organic fertilizers contain phosphorus in small quantities, in combination with nitrogen, potassium and organic acids:
| Type of organic fertilizer | Phosphorus content |
| Herbal compost → application of fertilizing | 0,45% |
| Prefabricated compost | 0,3% |
| Peat-manure compost → application of fertilizing | 0,2% |
| Vermicompost (vermicompost) → application of fertilizing | 2,5% |
| Humus → application of fertilizing | 0,48% |
| Horse manure → application of fertilizing | 0,29% |
| Mullein → application of fertilizing | 0,21% |
| Rabbit droppings → application of fertilizing | 0,59% |
| Bird droppings → application of fertilizing | 1,5% |
| Sapropel | 0,5% |
| Bone meal → application of fertilizing | Up to 35% |
| Fish bone meal | 4% |
Due to the high phosphorus content in bone meal, it is most often used for targeted feeding of plants with this element. However, it contains phosphorus in a water-insoluble form. This fertilizer belongs to the group of limitedly soluble ones.
“Bone meal can be used to amend the soil in the fall. If the acidity of the soil is high, by spring phosphorus will gradually pass into the soil solution, after which it will be absorbed by the plants. For liquid feeding, bone meal can be used together with a solution of potassium sulfate: 30 g of potassium sulfate per 10 liters of water and 50 g of bone meal there (see → use of potassium sulfate + reviews) Leave for several hours and water the plants.”
V. Trubin, gardener with 15 years of experience
Composts, vermicomposts, humus, and sapropel are valuable not only because they themselves contain a certain amount of phosphorus. They increase the activity of microorganisms that convert phosphates into soluble forms. These microorganisms include the bacteria Bacillus megaterium and fungi of the genus Penicillium.
Reviews on the effectiveness of phosphate fertilizers
Gardeners constantly use phosphorus fertilizers in one form or another. Numerous reviews speak about their effectiveness and impact on productivity:
“Without phosphorus there is no harvest - this is an axiom. I try to apply mineral fertilizers on my land very carefully, but I always use superphosphate. But not under the plants themselves, but when laying the compost heap. The compost turns out great! It contains no more nitrogen than is required for normal growth, and quite a lot of phosphorus. Abundant flowering and fruiting are ensured with such fertilizer” (Evgeniya, Vologda).
Current questions about phosphate fertilizers
Question No. 1. Do mineral phosphorus fertilizers spoil the soil?
Any mineral fertilizers spoil the soil. Therefore, they must be used in strict accordance with the instructions.
Question No. 2. Is it possible to do without phosphorus fertilizers in the non-chernozem region? (click to expand)
In one form or another, any gardener adds phosphorus to the soil, even if he does not use mineral salts. You need to look at the yield and the land. If the soil has a good structure, contains a lot of humus, and produces a healthy harvest, there is no point in pouring salt into it. To maintain its fertility, ordinary organic matter and EM preparations will be sufficient.
Rate the quality of the article. We want to be better for you:
Signs of element deficiency in the soil
To know whether it is worth adding phosphorus complexes to the soil, you need to know the symptoms of element deficiency in plants. By the appearance of the crop, you can determine the deficiency of a chemical element:
- The leaves change color. At first they become dark green and then acquire a dark purple color.
- Leaf blades grow irregularly shaped and fall off.
- Dark spots appear on the underside of the leaf.
- Reduced yield.
- Lack of natural phosphorus found in the soil.
- The roots take on a lilac color.
- The plant does not gain the required growth.
- Insufficient development of the root system.
See also
Classification and description of types of mineral fertilizers and their use in the garden
Read