Loading…
Loading…
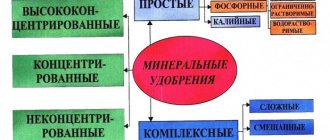
Growing vegetables, herbs and fruits is accompanied by comprehensive care, an obligatory part of which is fertilizing with fertilizers containing nitrogen. Let's figure out how saltpeter differs from urea, and how to use them correctly when caring for different crops.
Urea and saltpeter - are they the same thing or not?
These are two different fertilizers, but they share the following characteristics:
- Composition - both preparations contain nitrogen compounds.
- Peculiarities of impact: rapid growth of green mass by plants.
- Application results: increased yield.
Since urea is organic, and nitrates are inorganic, these products differ in the method of application. For example, organic matter is applied both by root and foliar methods. And inorganic compounds are only added to the soil. There are also several other significant differences between them. Therefore, we can definitely say that ammonium nitrate is not urea.
Urea: composition, types, application
Urea is the common name for the organic fertilizer urea (chemical formula: CH4N2O). The composition contains the maximum amount of nitrogen (compared to all other products), so urea is considered one of the most effective drugs.
Urea is a white crystalline powder that is highly soluble in water and ammonia (ammonia). There are no other varieties. Those. chemically and physically, urea always has the same stable composition. At the same time, ammonium nitrate differs from urea in different contents, for example, sodium nitrate, potassium nitrate, ammonium nitrate and others.
Urea is produced in the form of white spherical granules
This remedy is used in different cases:
- As a fertilizer to saturate the soil with nitrogen. This is especially important during the period of active growth: spring - first half of summer. Applying nitrogen fertilizers in July, August or autumn is impractical and can even harm the plants.
- Prevention of the spread of diseases and pests - adult plants and seedlings are often sprayed with urea solution.
- Increasing productivity by accelerating growth processes.
- Delayed flowering, which is especially important in late spring (flowers may freeze).
Important! Urea contains up to 46% nitrogen (by mass fraction). If plants lack this microelement, it is advisable to use urea.
Saltpeter: composition, types of application
Nitrates are called nitrates of various metals of the general composition XNO3, where X can be potassium, sodium, ammonium and other elements:
- sodium (NaNO3);
- potassium (KNO3);
- ammonia (NH4NO3);
- magnesium (Mg(NO3)2).
The product is also available in the form of mixtures, for example, ammonium-potassium nitrate or lime-ammonium nitrate. The complex composition has a more effective effect on plants, saturating them not only with nitrogen, but also with potassium, magnesium, calcium and other trace elements.
Fertilizer is used as one of the main sources of nitrogen. It is also applied at the beginning of the season for the following purposes:
- Acceleration of green mass gain.
- Increased yield (ripening dates may occur earlier).
- Light soil acidification, which is especially important for alkaline soils with pH = 7.5-8.0.
Important! Ammonium nitrate (ammonium nitrate) is practically not sold for private households.
This is an explosive substance that requires special conditions for transportation and storage. However, other nitrates can be found in the public domain.
In appearance, ammonium nitrate is practically no different from urea
Replacing fertilizers containing nitrogen
Sometimes gardeners and gardeners have a question about what can replace nitrogen fertilizers. Their use on acidic soils is limited; in some countries it is quite difficult to find saltpeter.
Urea is primarily used as a replacement for nitrate. In its absence:
- ammonium sulfate;
- ammonium chloride;
- any other types of saltpeter.
If you need to find a fertilizer that can replace urea, use any of the above.
To grow a rich harvest or achieve magnificent flowering in the garden, you should wisely select and use universal fertilizers. This will allow you not to harm the soil and provide the plants with proper care. Knowing the difference between urea and saltpeter, it is easy to make a choice.
What is the difference between urea and nitrate
Despite the fact that ammonium nitrate and urea are fertilizers of the same class (nitrogen), there are several differences between them. To find out what the difference is between them, you need to compare some characteristics.
By composition
There is a fundamental difference in composition between urea and ammonium nitrate. The first fertilizer is organic, and nitrates are inorganic substances. In this regard, the methods of their use, the speed of exposure and the permissible dosage differ from each other.
In terms of nitrogen content, urea is better than saltpeter: the latter contains up to 36% nitrogen, and urea - up to 46%. At the same time, urea always has the same composition, and nitrates are a group of inorganic substances that, along with nitrogen, include potassium, magnesium, sodium, calcium and other trace elements.
Effect on soil and plants
Organic fertilizer (urea) is absorbed more slowly by the plant. The fact is that only inorganic substances in the form of ions penetrate into the roots (they are highly soluble in water and have small molecular sizes). And the carbamide molecule is much larger. Therefore, the substance is first processed by soil bacteria, and only then does nitrogen penetrate into the plant tissue.
Nitrates already contain nitrates - negatively charged NO3 ions - small molecules that quickly penetrate the root hairs along with water. Therefore, the fundamental difference between urea and ammonium nitrate is that organics act more slowly, and inorganics act noticeably faster.
Important! Urea has a longer action compared to nitrates.
It will supply the plants with nitrogen for weeks on end.
By application
The methods of using these fertilizers are also different:
- Nitrates (inorganics) can only be applied by the root method, i.e. dissolve in water and pour under the root. The fact is that saltpeter does not penetrate through the leaves, and there is no point in spraying plants.
- Urea (organic matter) can be applied both by root and foliar methods, alternating both. Organic compounds penetrate well through leaf tissue. And in the soil they first turn into inorganics, after which they are absorbed by the root system.
Organic nitrogen fertilizers can be applied foliar
Nitrogen fertilization
Urea or urea (CO(NO2)2) crystalline granules with a high nitrogen content. An effective water-soluble fertilizer with a number of positive properties.
- The nutritional properties of 1 kg of urea are equivalent to 2.2 kg of ammonium sulfate or 3 kg of sodium nitrate.
- The chemical formula of the substance allows the fertilizer to have a beneficial effect on the soil.
- For use on sandy and sandy loam soils, urea is much better, in contrast to ammonium nitrate.
- Nitrogen is in a compound that is easily soluble in water, and the nutrients are not washed into the underlying soil.
- Recommended for use as foliar feeding. If the proportion is observed, it has a mild effect and does not burn plant leaves.
Urea contains 46.2% nitrogen and can be used to add to the soil when digging. The crystals are deepened 4–5 cm into the soil to avoid ammonia evaporation. When sowing seeds, urea with potassium fertilizer is placed in the furrows, sprinkled with soil a few centimeters and the seeds are sown. During the active growth of the green part of the plant, root and leaf feeding of agricultural and flower crops is carried out. A 5% urea solution is recommended for spraying leaves.
Urea is a universal soft fertilizer for gardening. Recommended for feeding potatoes, garlic, cucumbers, berries, shrubs, roses and other garden and house flowers.
Which is better: saltpeter or urea
Both fertilizers (urea and ammonium nitrate) have their pros and cons, so it is difficult to say unambiguously which one is better. For example, urea has the following benefits:
- Increased nitrogen content - at least 10%.
- Lack of explosion hazard (compared to ammonium nitrate).
- Can be applied both by root and foliar methods.
- The effect is long-lasting, can be used 1-2 times per season.
- Does not increase acidity.
- Does not cause burns on the surface of leaves, stems and flowers, even with foliar application.
The disadvantages of this feeding include:
- Slow action - the effect is noticeable only after a few weeks.
- Fertilizing can be applied exclusively in the warm season, since it does not penetrate frozen soil.
- It is not recommended to embed in the soil in which the seeds are planted (for example, for seedlings) - their germination may decrease.
- Organics are not allowed to be mixed with other fertilizers. They can only be entered separately.
Benefits of saltpeter:
- Can be used both in the warm season and in the fall, for the winter.
- Increasing acidity is beneficial for some plants, as well as for alkaline soil.
- It is quickly absorbed by plants, the result is noticeable almost immediately.
- Destroys weed leaves, so it can be used in a tank mixture with various herbicides. However, spraying must be carried out with caution so as not to get on the leaves of the crop (for example, before emergence in the spring).
- Can be applied in mixtures with other fertilizers.
Flaws:
- Ammonium nitrate is an explosive substance.
- It increases the acidity of the soil, which for other plants (and especially for acidic soil) can be a significant disadvantage.
- There is less nitrogen, therefore, the consumption of the substance for the same area is greater.
- If you accidentally touch leaves or other green parts of the plant while watering, it may cause a burn.
Important! Up to 70% of the applied nitrogen is consumed by various microorganisms in the soil. Despite the fact that urea contains only 10% more nitrogen than ammonium nitrate, organics are better than inorganics in this regard.
Nitrogen compounds contribute to the rapid development of plants
You can use urea fertilizer instead of ammonium nitrate. Organic matter does not change the soil environment; it is recommended to apply it at the root or spray the green part of the plants with the solution. But if you need to achieve a quick effect, it is preferable to use inorganic nitrates.
Which is better for wheat: urea or saltpeter
For winter wheat varieties, saltpeter is often used. The choice is due to the fact that it is absorbed even in frozen soil. Under similar conditions, the use of urea will be ineffective. In fact, it will lie in the ground until the next season, and only after processing by bacteria will it begin to enter plant tissue through the root system.
Adding ammonium nitrate to the soil
The application rates for any fertilizer depend on the instructions on the package, climatic conditions, crops grown, as well as the type and condition of the soil. On poor, depleted soil, 40–50 g per 1 square meter is recommended. meter of soil. On fertile, well-cultivated soil, 20–30 g per meter is sufficient. Used when digging up a vegetable garden or when planting seedlings in open ground (1 tablespoon of fertilizer for each plant).
When planting root crops (potatoes, beets, etc.), fertilizing is carried out 3–4 weeks after the appearance of green shoots. Shallow grooves are created between the rows. Ammonium nitrate is added to the prepared recesses at the rate of 6–8 g per 1 square meter. meter of soil. Sprinkle with 2–3 cm of soil. Fertilizer is used once a season.
Vegetables (cucumbers, tomatoes, etc.) are fed when planting seedlings or 7–10 days after transplanting. After using the fertilizer, the plant grows stronger and begins to grow green mass. The second feeding is carried out about a week before flowering.
During the period of fruit formation, nitrogen fertilizers are not used.
In early spring, when the buds bloom and leaves appear on fruit trees, the soil is treated with ammonium nitrate. A one-time root feeding is carried out by distributing dry matter at a rate of 15–20 g. per 1 sq. meter of soil. When the soil is moistened, nutrients are transported with moisture to the root system of trees. Fruit trees are watered with an aqueous solution (30 g per 10 liters of water) 2-3 times a season.